Photoelectrochemical system based on Cu2O Photocathodes
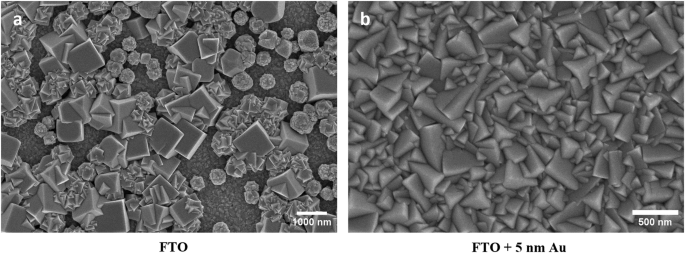
Electrodeposited Cu2O film functions as the component that absorbs light, generating photogenerated electrons used for solar-assisted water splitting. Typically, transparent conducting oxide films (FTO) are employed as substrates in transparent electronic devices. The substrate’s contact with Cu2O plays a pivotal role in creating an ohmic junction for the collection of photogenerated holes. For example, Cu2O and FTO contact doesn’t perfectly form an ohmic junction but rather creates a Schottky barrier, hindering hole collection and negatively affecting the current-potential response25. Furthermore, the Cu2O morphology when deposited on bare FTO differed notably, forming large Cu2O crystals compared to the consistent, uniform, and dense growth on gold-coated substrates. Figure 1 illustrates this variation across substrates, where it seems that the Au substrate impacts both the electronic junction and the electrodeposited film quality. We theorized that thin Au layers might address these issues while retaining high transparency. As depicted in Fig. 1, sputtering with an equivalent dose of 5 nm Au formed an island-like coating on the FTO. Interestingly, Cu2O’s electrodeposition onto these Au-coated substrates yielded films similar to those on thicker Au layers (10 and 15 nm), unlike the large Cu2O particles on bare FTO (Supplementary Fig. 1).
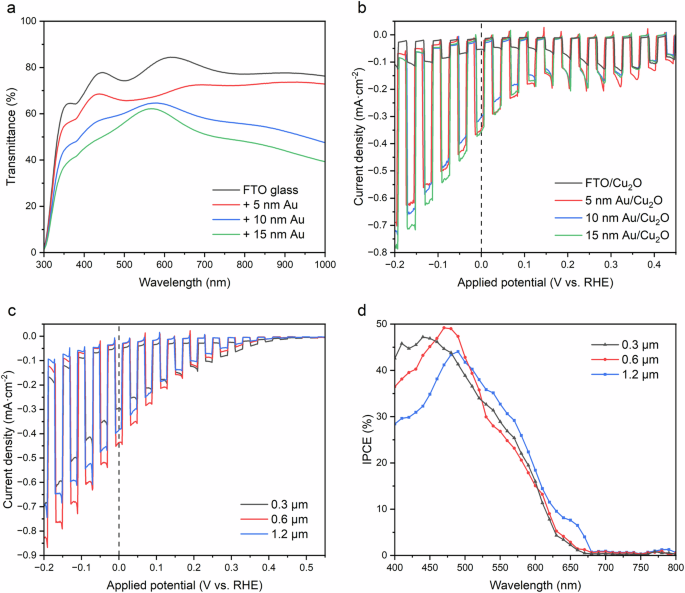
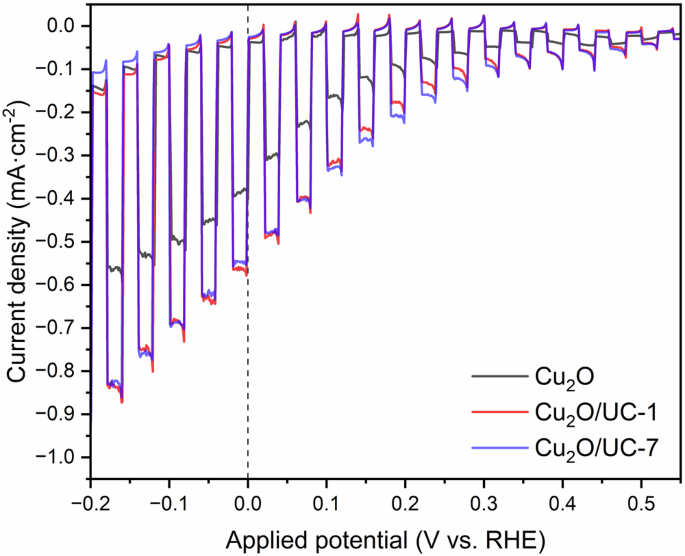
The effects of Au thickness on photoelectrochemical and optical properties are shown in Fig. 2a, b. All samples were treated the same in terms of deposition of Cu2O, where a 60-minute experiment was performed to obtain approximately 600 nm of cuprous oxide layer. The only difference was the various Au underlayer thicknesses. As seen in Fig. 2b, the photocurrent does not change significantly upon increasing the thickness of the gold layer. The maximum photocurrent is approximately −0.35 mA·cm−2 for all three cases (5, 10, and 15 nm) with minor variations obtained at the applied potential of 0 V vs. RHE. The difference is visible when we increase the applied potential to a higher value, where the highest photocurrent was observed in ascending order of Au thickness, namely on a thicker layer of Au (−0.7 mA·cm−2). In addition, the major distinction that draws our attention in this graph is the importance of the gold layer: as already mentioned earlier, Cu2O and FTO contact doesn’t perfectly form an ohmic junction which negatively affects the current-potential response.
a Transmittance spectrum of FTO substrates with varying amounts of sputtered Au. b Photoelectrochemical performance of 60 min electrodeposited Cu2O on various Au-treated FTO substrates under 1-sun chopped illumination. c Photoelectrochemical performances and d IPCE spectra for Cu2O films with different thicknesses deposited onto 5 nm Au-treated FTO substrates.
The thickness of the Cu2O photoactive layer is the next parameter to examine. Previous publications utilized Cu2O films of 500 nm or larger6,7,15. Transparency was critical in our study, thus we investigated a range of Cu2O thicknesses by altering the time of electrodeposition onto FTO substrates coated with 5 nm of Au. Three durations were investigated, with electrodepositions lasting 120, 60, and 30 minutes, generating film thicknesses of approximately 1200, 600, and 300 nm, respectively. The thickness of the films was measured by the profilometer (Dektak XT, Bruker). The photocathode performances of the devices varied, as shown in Fig. 2c, where the photoelectrode with the thinnest absorber layer (300 nm) demonstrated reduced photocurrents, whilst the 600 and 1200 nm films had virtually equal responses. The associated incident photon-to-current conversion efficiency (IPCE) is altered by variations in absorber thickness. According to Fig. 2d, the lower energy photons (~500−600 nm) are poorly absorbed by these Cu2O films. For significant absorption in this range, film thicknesses of several micrometers are required26 but for our configured tandem device, long-wavelength photon transmittance is an important factor.
Light penetration depth and carrier diffusion length are two key properties of semiconductors utilized either in photovoltaic or photoelectrochemical applications. The light penetration depth of Cu2O at λ = 600 nm is approximately 2.2 μm27. The mobility of the charge carriers, which is heavily influenced by the synthesis procedure, determines the carrier diffusion length. For example, a single crystal or polycrystalline Cu2O produced at high temperatures (thermal oxidation or sputtering) can attain a high carrier mobility of 100 cm2·V−1·s−128,29. Whereas the electrodeposition (a technique that was implemented in this work) produces tiny Cu2O grains and carrier mobilities that rarely surpass 5 cm2·V−1·s−130. The carrier diffusion lengths obtained in high mobility Cu2O systems (~100 cm2·V−1·s−1) fall within the 1–10 μm range, which is in good agreement with the depth of light penetration31. High efficiencies are made possible in this situation by the ability to absorb almost all photons with energy higher than the bandgap. Lower mobility Cu2O-based systems (>5 cm2·V−1·s−1) result in charge carrier lengths much below 1 μm, which reduces the efficiency of the device by preventing the collection of longer wavelength (>600 nm) photons32. As a result, the 60-minute electrodeposition photocathode was chosen as a comparatively optimum choice for balancing PEC performance and optical transparency in the tandem water-splitting system.
UC/Cu2O tandem device
UC devices typically consist of a material that absorbs low-energy photons, such as infrared light, and emits higher-energy photons in response. These devices can be integrated with solar water-splitting materials to increase the overall efficiency of the system. One example of such integration is illustrated in Fig. 3b, where PbS nanocrystals (NCs) absorb the low-energy photons, and by the TTA process, the rubrene and bathocuproine (BCP) allow to combine low-energy photons and produce the higher one (visible light). These high-energy photons are reflected by the silver mirror and get absorbed by the photocatalyst from the back side. Figure 3b displays a schematic illustration of the fully constructed tandem device composed of a Cu2O photoactive layer and an upconversion device. In this configuration, the two systems are positioned in a back-to-back arrangement, namely our photocatalyst will be irradiated with visible light from dual perspectives (front and back-side illumination). A dual-absorber UC/Cu2O tandem system utilizes photons from distinct parts of the solar spectrum in order to efficiently capture a wide range of sunlight.
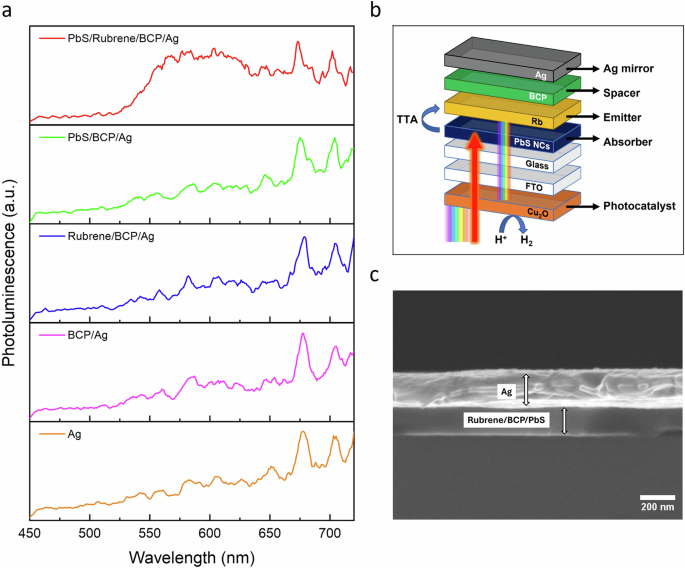
a Photoluminescence of UC device with control groups. Inset: control groups consist of the same components with the elimination of one or several elements. The devices were excited at 960 nm. b Schematic representation of UC device/Cu2O configuration. c Cross-sectional scanning electron microscopy (SEM) image of assembled UC device on a silicon substrate.
Regarding the UC device itself, starting from the first layer, PbS NCs were deposited on the glass via the spin coating method in a nitrogen-filled glovebox. Without exposing it to air, the samples were loaded into a thermal evaporation instrument, where rubrene, BCP, and silver were deposited on top of PbS film, respectively. Figure 3b illustrates the layers consisting of PbS, rubrene, BCP, and Ag covered from both sides with glass and encapsulated with epoxy glue to prevent air and moisture contact. PbS nanocrystals absorb low-energy photons, namely at 960 nm (Supplementary Fig. 5), therefore, the device was illuminated with 960 nm incident light and upconverted photoluminescence (PL) was measured. Figure 3a and Fig. 4 display the upconverted photoluminescence (PL) intensity of the device at λ~550–625 nm. It was demonstrated that each component is crucial for obtaining the upconversion phenomenon since the elimination of one or several elements does not produce the same effect. It is possible to assume that two sharp peaks that appeared at 675 and 700 nm are due to the Ag mirror as those peaks appeared in all tested samples. Given that Cu2O exhibits photoactivity within the visible spectrum up to 600 nm (Fig. 2d), the emitted photons from the UC device have a chance to get absorbed by this photoactive material.
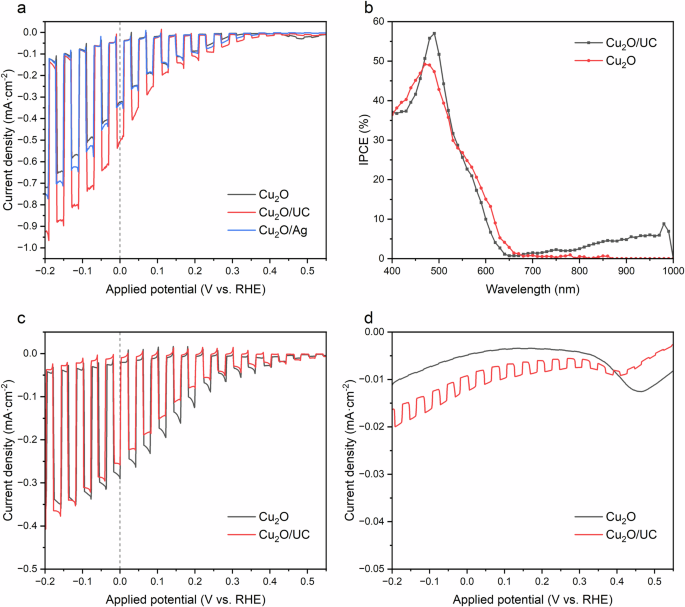
We hypothesize that integrating upconversion device with Cu2O photocathode would enhance the absorption ability of Cu2O which in turn enhances the photoelectrochemical performance. The photoelectrochemical performance was measured by monitoring the photocurrent changes in the device. Figure 5a illustrates that Cu2O coupled with an upconverter (UC) performs better than bare Cu2O in terms of produced photocurrent density, namely bare Cu2O produces −0.32 mA·cm−2 at 0 V (vs. RHE) whereas Cu2O/UC exhibits a peak at −0.5 mA·cm−2 demonstrating an enhancement of 56%.
a Photocurrent responses and corresponding b IPCE of photocathodes, produced by 60 min electrodeposition of Cu2O on 5 nm Au layer, tested in Na2SO4 (pH 6.4) electrolyte under 1-sun illumination. c Photocurrent responses of photocathodes under visible light. d photocurrent responses of photocathodes under IR light (950 nm).
Relying on these statements and data, one can say that the observed effect may happen due to the presence of a thick silver layer that functions as a mirror. To eliminate such concerns, we fabricated the device consisting of Cu2O and Ag by removing the upconversion part (PbS, Rubrene, BCP). As shown in Fig. 5a, the Ag does not affect the improved photocurrent meaning that our idea worked and confirmed. In addition, Fig. 5b shows the variations in spectrum response of the devices under monochromatic illumination as IPCE spectra. These two thin Cu2O films use photons of higher energy (wavelengths shorter than 600 nm), as shown by an inflection in the IPCE spectra. The difference between these two devices is the response to the near IR region, where Cu2O coupled with an upconversion device clearly utilizes the photons in this range. Figure 5d was constructed to accurately demonstrate the effect of upconversion on photocurrents produced by Cu2O. It can be seen that under non-concentrated 950 nm illumination (LED source, 400 µW·cm−2) Cu2O coupled with an upconversion device shows some photoresponse compared with bare Cu2O. As for the photoactivity of photoelectrodes under visible light (LED source, 390–730 nm), no major difference was observed (Fig. 5c).
To demonstrate the enhanced performance of our device, Table 1 was constructed by compiling current reports of similar UC-PEC water-splitting systems based on Cu2O. There are few documented works that use such configuration (Cu2O/UC), and even when they do, they all rely on upconversion materials based on lanthanides. There are two primary limitations that affect the PEC efficiency of these systems. One issue is that the upconversion efficiency of near-infrared in rare earth-doped NaYF4 is low, as these materials can only utilize specific wavelengths33. That is why photoelectrode systems listed in Table 1 illustrate the photoactivity using concentrated laser beams (mostly 980 nm). In one of the systems, namely NaYF4:Yb–Er/AZO/Cu2O, researchers tried to test under solar light (Xe lamp), however, no improvement in terms of photocurrent was observed. Another issue is that the high electrical resistance of NaYF4, the near-infrared upconversion material employed in the PEC water-splitting system, creates a significant obstacle for the photo-carriers as they traverse the UC layer towards the conductive substrate. Consequently, this leads to a substantial decrease in the PEC water-splitting efficiency.
In our system, the two above-listed issues are eliminated. As can be seen in the main text, our device can operate under solar irradiation (100 mW·cm−2) and even low-power near-infrared light sources, which is more applicable to real-world scenarios. In addition, since our upconversion device is constructed in a way that does not physically contact the conductive substrate and photocatalyst, it will not act as a barrier for charge carriers.
At this point, it should be mentioned that there are two limitations present in this work related to photocatalyst and upconversion devices. First, due to the focus on designing and optimizing the photoelectrode system to enhance the light-harvesting capability of Cu2O, the improvements of the photocathode itself in terms of photocurrent and stability were neglected. Chemical instability (photocorrosion) and low photocurrents are common issues for the bare Cu2O-based photocathodes which limit the measurement of hydrogen production and solar-to-hydrogen efficiency.
Second, due to the instability of the main material, it is difficult to estimate the stability of the upconversion device. As can be seen in Fig. 6, our fabricated upconversion device is stable for a prolonged time sitting on the bench under normal lab conditions (air atmosphere, room temperature) since it was encapsulated preventing air or moisture exposure. It should be mentioned that rubrene can undergo degradation when exposed to extended light, particularly ultraviolet (UV) radiation34. Nevertheless, due to the presence at the top of Cu2O, which absorbs UV-visible light, the rubrene is shielded from photodegradation. Therefore, improvements in electrode stability and solar-to-hydrogen efficiency are anticipated in future works.