Structure and properties of pseudohalide–MFPs
Here, we incorporated the heavy elements (Cl, Br) into MDABCO2+ and investigated the band nature evolution and radiation detection performance of emerging pseudohalide–MFPs (MDABCO–Cl/Br)–NH4(BF4)3 due to the MDABCO–NH4(BF4)3 system is easy to manipulate at the molecular level (Fig. 1c). The electrostatic potential mapping reflects the organic molecule’s characteristics post-design, wherein halogenation induces a redistribution of electron density, resulting in a positive charge on the remaining part of the molecule and a negative charge on the halogen side. Correspondingly, the structural evolution information is evaluated by the tolerance factor τ, which ensures that the crystal structure is stable with the appropriate dimensions (Fig. 1c)23,24.
The (MDABCO–Cl/Br)–NH4(BF4)3 single crystals were prepared at room temperature (~25 °C). Transparent crystals can be obtained by carefully controlling the evaporation of solvents from aqueous solutions containing HBF4 (Supplementary Fig. 1). The scanning electron microscope (SEM) analyses confirmed the presence of flat crystal cutoff surface (Supplementary Fig. 2). Firstly, the crystal structure information is revealed by single-crystal X-ray diffraction (XRD). Crystal structure analyses disclose that the space group P21 with monoclinic system changes to P1 with triclinic after inserting the C–Cl/Br covalent bond. The density also gradually increases (from 1.728 g cm−3 for (MDABCO)–NH4(BF4)3 to 1.950 g cm−3 for (MDABCOBr)–NH4(BF4)3). In detail, the three pseudohalide–MFPs all exhibit three-dimensional structures. (MDABCO–Cl/Br)2+ cation is centrally located within a cage-like NH4(BF4)6 polyhedra structure. The hydrogen bond between C–H···F, C–H/Cl···F and C–H/Br…F is responsible for the stabilization of the (MDABCO)–NH4(BF4)3, (MDABCOCl)–NH4(BF4)3, and (MDABCOBr)–NH4(BF4)3 components, respectively (Fig. 1d and Supplementary Fig. 3–5)25,26.
The properties of pseudohalide–MFPs semiconductors are intricately linked to the band gap; therefore, we employ density functional theory (DFT) to compute the band structure. As shown in Fig. 1e, the band gap of (MDABCO)–NH4(BF4)3 is 7.4 eV, which closely aligns with the reported for other analogous compounds18. Interestingly, there was a reduction in the band gap to 6.6 eV for (MDABCOCl)–NH4(BF4)3 and 5.5 eV for (MDABCOBr)–NH4(BF4)3, respectively, after heavy atom modification. The reported pseudohalide–MFPs band gap presented here is believed to be the smallest known to date. The dominance of the change in the band gap is undoubtedly attributed to the fine-tuning of the structure. The Hirshfeld partition of molecular density (IGMH) is employed herein to analyze diverse chemical bonds and interactions in pseudohalide–MFPs27. Fig. 1f and Supplementary Fig. 6 illustrate the presence of covalent bonds (C–C, B–F), hydrogen bonds (C–H…F), and steric hindrance effect within spherical molecules in pseudohalide–MFPs. Meanwhile, the presence of highly electronegative Br and Cl atoms leads to a more refined van der Waals intermolecular interaction in (MDABCOBr)–NH4(BF4)3 and (MDABCOCl)–NH4(BF4)3.
The interactions between these components are expected to influence the structure coupling. The heightened electrostatic potential difference between (MDABCO–Cl/Br) and NH4(BF4)6 enhances the strength of hydrogen bonding compared to MDABCO2+, and the additional van der Waals forces acting upon also facilitate the coupling between (MDABCO–Cl/Br) and NH4(BF4)6 pseudohalide–MFPs. It can also be intuitively seen from the Mulliken charge population diagram that MDABCOBr2+ and MDABCOCl2+ have higher inductive effects on anion groups, especially the former (Fig. 1g)28. The charge density distribution of pseudohalide–MFPs in Supplementary Figs. 7–9 provides further evidence, revealing a higher occurrence of charge deletions in the conduction band minimum (CBM) and valence band maximum (VBM) for (MDABCO)–NH4(BF4)3 compared to (MDABCOCl)–NH4(BF4)3 and (MDABCOBr)–NH4(BF4)3 crystals, suggesting its stronger electronegativity (yellow indicates charge deletions while blue represents charge aggregation).
Considering these, the band gap reduction in (MDABCO–Cl/Br)–NH4(BF4)3 can be easily comprehended. Specifically, the intermolecular interactions in pseudohalide–MFPs dominate the frontier orbital separation of organic molecules, as well as orbital coupling with NH4(BF4)6 octahedra29. As shown in Supplementary Fig. 10, the pseudohalide–MFPs conduction band (CB) is mainly composed of organic molecules, including C-s, N-p, and H-s orbital hybrids. By contrast, the Cl-s orbital is observed in (MDABCOCl)–NH4(BF4)3, and there is a clear overlap with the N-p orbital, which tailed CBM to a lower energy state10. Based on the aforementioned analysis, it can be attributed to the electron delocalization resulting from the significant electronegativity difference in DABCOCl2+ and the stronger interaction within the (MDABCOCl)–NH4(BF4)3. Interestingly, the CB of (MDABCOBr)–NH4(BF4)3 is composed of two main parts, and the situation of the latter part is similar to that of (MDABCOCl)–NH4(BF4)3. In contrast, the former part emphasizes the hybridization between C-s and F-p orbitals, thereby elucidating that the modulation of band gap primarily arises from intricate intermolecular interactions.
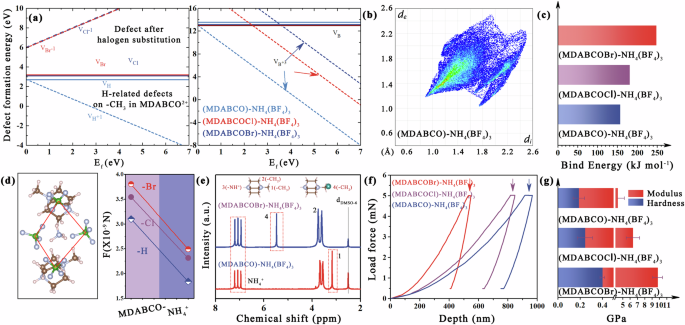
Ion migration driven by the high electric field in radiation detection can significantly deteriorate electrical properties. This situation is particularly exacerbated in halide–MFPs, primarily due to the considerably lower ion migration barrier18. However, the migration process of BF4− reveals that including a rotational process in its movement dramatically increases the ion migration barrier in pseudohalide–MFPs (Fig. 1h). The results also show that structural adjustment can effectively improve the ion migration barrier of pseudohalide–MFPs. Defect formation in pseudohalide–MFPs was further carried out. The DFT calculation reveals that H atom on the –CH3 in MDABCO2+ are readily formed point defects VH at CB and VB, as well as charge defects VH+1 at VB (formation energy ~2.7 eV). Once the H atom is replaced by Br/Cl, it leads to an increase in the formation energy of VBr and VCl, reaching 3 eV. Additionally, the charge defect VBr−1 and VCl−1 experience a rise up to 6 eV due to the aforementioned intermolecular van der Waals interaction (Fig. 1f). Unlike halide–MFPs, X-site defects in pseudohalide–MFPs are difficult to form because they involve overcoming both covalent B–F bonds and intermolecular hydrogen bonds. The simulation results indicate that the formation energy (VB and VB+) of all three compounds >10 eV, particularly for (MDABCOCl)–NH4(BF4)3 and (MDABCOBr)–NH4(BF4)3, rendering their formation nearly impossible. Still, this increasing trend also signifies a gradual reinforcement of hydrogen bonding (Fig. 2a).
a Defect formation energy of pseudohalide–MFPs. b Visualization map of the distribution of the interactions and the 2D fingerprint plots of (MDABCOBr)-NH4(BF4)3. c Calculated binding energies between MDABCOBr2+-NH4(BF4)6, MDABCOCl2+-NH4(BF4)6, and MDABCO2+-NH4(BF4)6. d The Coulomb interactions of pseudohalide–MFPs. e 1H NMR spectra of pseudohalide–MFPs. f Load-force-dependent indentation depth curve of different pseudohalide–MFPs. g Hardness and Modulus comparison of different pseudohalide–MFPs.
The subsequent investigation examines the impact of structural modulation interaction on the stability of pseudohalide–MFPs. We present a visualization of the distribution of interactions using Hirshfeld surface analysis30,31. The prevalence of blue and white regions in all three pseudohalide–MFPs suggests that weak interactions between cations and anions dominate the system (Fig. 2b, Supplementary Figure 11). The map simultaneously illustrates that (MDABCOCl)–NH4(BF4)3 and (MDABCOBr)–NH4(BF4)3 exhibit a larger surface distribution area, attributable to their more interaction. The ultimate result is that the components in (MDABCOCl)–NH4(BF4)3 and (MDABCOBr)–NH4(BF4)3 exhibit heightened binding energy (lower binding energy means easier dissociation), and the coulomb forces between the anions-cations, thereby attaining optimal stability under same conditions, especially the (MDABCOBr)–NH4(BF4)3 (Fig. 2c, d). Also, the observation of H(-NH4+) signals toward lower chemical shifts in the 1H NMR spectra for (MDABCOBr)–NH4(BF4)3 also serves as evidence for enhanced hydrogen bond strength associated with binding energy (Fig. 2e). As shown in Supplementary Fig. 12, the decomposition temperature measured by thermos-gravimetric analysis also confirms this trend. The thermal decomposition of (MDABCOBr)–NH4(BF4)3 mainly occurred at ~220 °C, higher than that of the other two analogs.
Crystal stability is also intricately linked to the mechanical indices related to lattice stiffness. The mechanical properties of the (001) plane were evaluated through a nanoindentation measurement. The load-force-dependent indentation depth curve of pseudohalide–MFPs crystal in Fig. 2f provides elastic and plastic deformation information of the sample under the nanotip. It indicates that the indentation depth varies across different single crystals when subjected to identical loads32,33. The indentation hardness and Young’s modulus of the single crystals are presented in Fig. 2g. The order of (MDABCO)–NH4(BF4)3 < (MDABCOCl)–NH4(BF4)3 < (MDABCOBr)–NH4(BF4)3 can be attributed to stronger hydrogen bonds and other interactions in (MDABCOBr)–NH4(BF4)3. Further DFT calculations confirmed the mechanical properties, including hardness, Young’s modulus, Poisson’s ratio, and shear modulus. The data extracted using Elastic-post software are listed in Supplementary Table 134. The representative three-dimensional and two-dimensional plots are shown in Supplementary Figs. 13 and 14, and it can be seen that the trend of contour lines is consistent with the experimental measurement.
X-ray detection and imaging
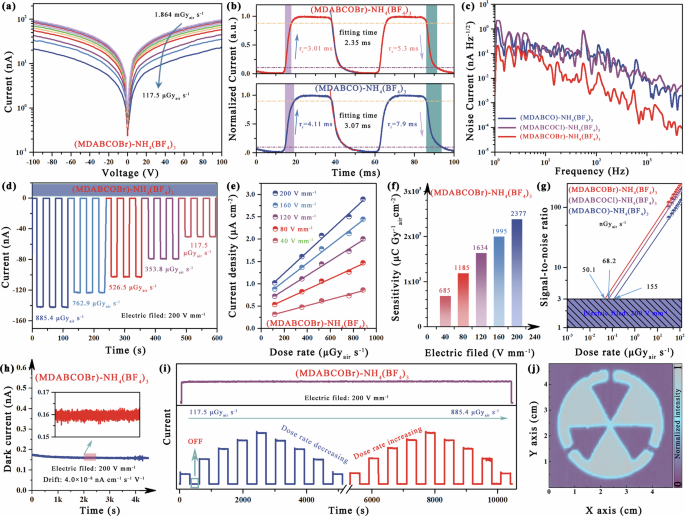
Then, the electrical and X-ray detection properties of the pseudohalide–MFPs single crystals were evaluated. Due to the incorporation of large electronegative elements, the dielectric constant (εr) of (MDABCOCl)–NH4(BF4)3 and (MDABCOBr)-NH4(BF4)3 surpasses that of (MDABCO)-NH4(BF4)3 (Supplementary Figure 15). A larger εr implies a weakened shielding effect on charges, facilitating their separation35. As shown in Supplementary Figure 16a, we calculated the X-ray absorption coefficients of pseudohalide–MFPs and commercial Si semiconductors at photon energies ranging from 1 keV to 10 MeV. The introduction of the Br element in (MDABCOBr)–NH4(BF4)3 significantly increases its absorption coefficient, particularly surpassing that of Si within the energy range of 15–200 keV. Supplementary Figure 16b displays the thickness of the MFPs single crystals, enabling it to absorb 100% of X-ray energy at 20 keV. On this basis, an Au/pseudohalide–MFPs crystal/Au planar X-ray detector is constructed. Fig. 3a shows the current–voltage (I–V) curve for the (MDABCOBr)–NH4(BF4)3 device at different dose rates. The charge collection efficiency is related to the carrier mobility µ and the carrier lifetime τ. High µτ products are preferred for outstanding-performance electronics. We derive the µτ product by fitting the dark conductivity shown in Supplementary Fig. 17 using a modified Hecht equation36. The resulting µτ value were 4.25 × 10−5 cm2V−1 for (MDABCOBr)–NH4(BF4)3, 8.36 × 10−6 cm2V−1 for (MDABCOCl)–NH4(BF4)3 and 1.43 × 10−6 cm2V−1 for (MDABCO)–NH4(BF4)3, respectively. A large µτ product typically results in a large device SNR, allowing the detector to resolve low dose-rate X-rays.
a The irradiated J–V curves of based (MDABCOBr)-NH4(BF4)3 X-ray detector under different dose rates. b Time response of (MDABCOBr)-NH4(BF4)3 and (MDABCO)–NH4(BF4)3 devices. c Noise current spectra of three pseudohalide–MFPs devices. d Photocurrent stability of (MDABCOBr)–NH4(BF4)3 device under different dose rates. e X-ray response of current density of (MDABCOBr)–NH4(BF4)3 device with various dose rates at different electric field. f The sensitivity of the (MDABCOBr)–NH4(BF4)3 device at different electric fields is obtained from (e). g The dose rate dependent SNR of the different pseudohalide–MFPs devices. h Dark current drift of (MDABCOBr)–NH4(BF4)3 device under the electric field of 200 V mm−1. i On/off and long-time operation stability of the (MDABCOBr)-NH4(BF4)3 device. j The X-ray image of (MDABCOBr)–NH4(BF4)3 device.
The response time of the X-ray detector plays a crucial role in practical applications as it directly impacts the detection speed. The response time was estimated using the transient current acquisition method. As shown in Fig. 3b and Supplementary Figure 18, the impulse response curves show a rise and fall time (from 10% to 90% of the saturated photocurrent) of 3.01 ms and 5.05 ms, respectively, for the (MDABCOBr)–NH4(BF4)3 device, which is faster than the other two analogs. In addition, the decay time information is obtained by fitting the impulse response curve, and the target device has a minimum decay time of 2.35 ms.The short response time is due to low defect density, fewer grain boundaries, and uniform lattice orientation. This advantage allows the device to obtain a more convenient charge transport path. For X-ray detection applications, noise is also an important figure of merit, which was evaluated with a fast Fourier transform signal analyzer37. Figure 3c demonstrates a significant reduction in noise for (MDABCOBr)–NH4(BF4)3 device compared to both (MDABCO)–NH4(BF4)3 and (MDABCOCl)–NH4(BF4)3. As mentioned above, this indicates an enhancement in lattice stiffness, effectively suppressing structural perturbations during detection.
The evaluation of X-ray detector performance relies heavily on the determination of sensitivity and detection limit. To assess sensitivity, we quantified the photocurrent response in terms of the on/off ratio under various dose rates and applied electric fields (Fig. 3d, Supplementary Figs. 19–21). The generated current density in each determined electric field exhibits a linear correlation with the X-ray dose rate, as shown in Fig. 3e. The (MDABCOBr)–NH4(BF4)3 device yield a sensitivity of 2377 μC Gyair−1 cm−2 at 200 V mm−1, which is 118 times higher than that of the commercial ɑ-Se detector (20 μC Gyair−1 cm−2@10,000 V mm−1) and represents a record value ever reported for MFPs devices (Supplementary Table 2). The sensitivity for (MDABCO)–NH4(BF4)3 and (MDABCOCl)–NH4(BF4)3 devices are 617 μC Gyair−1 cm−2 and 1522 μC Gyair−1 cm−2, respectively (Fig. 3f, Supplementary Figs. 19 and 20). While this is a modest gap to the performance of state-of-the-art metal halide X-ray detectors, our design principles establish that heavy atom design and structural optimization are key to driving the performance of pseudohalide–MFPs. According to the given definition, the detection limit fixed bias is defined as a dose rate that yields a signal-to-noise ratio of 333. Figure 3g illustrates that the lowest detection limit for (MDABCOBr)–NH4(BF4)3 device is 50.1 nGyair s−1, better than the other two pseudohalide–MFPs devices.
The deterioration of device performance caused by ion migration is regarded as a persistent ailment in metal halides. The current hysteresis serves as visual evidence for ion migration. Supplementary Figure 22 presents the dark current cycle curves of (MDABCO)–NH4I3 and (MDABCOBr)–NH4(BF4)3 devices in the −200 V and 200 V ranges. The (MDABCO)–NH4I3 exhibited a more pronounced hysteresis behavior attributed to severe halogen ion migration. Regarding device operation, migrating ions at the electrode-crystal interface should be cautioned, as it can undergo chemical reactions with the electrode, ultimately leading to its gradual failure38. We designed an immersion experiment to compare the corrosion of electrodes in pseudohalide–MFPs and halide–MFPs devices (see details in Supplementary Fig. 23). It can be seen that after immersing in (MDABCO)–NH4I3 dispersion solution for 72 h, the Cu film undergoes a noticeable darkening accompanied by a deteriorated coverage, and nearly disappears after soaking for 120 h, indicating its susceptibility to corrosion within this I-rich environment. Conversely, when immersed in the (MDABCOBr)–NH4(BF4)3 dispersion solution for 120 h, no discernible changes occur for the Cu film, indicating that electrode degradation in the pseudohalide–MFPs system is not related to the active material (Supplementary Fig. 24). This experimental conclusion is consistent with the results of previous simulations (Fig. 1h), which show that the disgusting ion migration in pseudohalide–MFPs is largely inhibited.
Further, we obtained the ion migration energy activation (Ea) of MFPs by fitting the temperature-dependent conductivity curve with the Nernst–Einstein equation39. The results also indicate that the activation energy (Ea) of pseudohalide–MFPs is greater than that of halide–MFPs devices, which aligns with the results from the simulation experiments. Meanwhile, the careful structural design of (MDABCOBr)–NH4(BF4)3 devices enables the highest ion migration energy in the pseudohalide–MFPs device (Supplementary Fig. 25 and Fig. 1h).
Then, the operating stability of (MDABCOBr)–NH4(BF4)3 devices is evaluated. As shown in Fig. 3h, the device operates over 4000 s at an electric field of 200 V mm−1 with a dark current drift only 4.0 × 10−8 nA cm−1 s−1 V−1, which is lower than reported value for analog single crystals device ((MDABCO)–NH4(PF6)3, 3.35 × 10−6 nA cm−1 s−1 V−1)18. Impressively, the detector exhibited a stable X-ray on/off response at different dose rates even after 10,000 s, and maintained its photocurrent signal without decay under continuous X-ray irradiation for an extended period of time (Fig. 3i). The stability of (MDABCO)–NH4I3 was compared under the same operating conditions. The device exhibited significant dark current fluctuation during long-term operation, with a drift value of 2.66 × 10−8 nA cm−1 s−1 V−1, which is two orders of magnitude higher than the (MDABCOBr)–NH4(BF4)3 devices. Additionally, the device also showed significant instability under X-ray irradiation, which differs from the target device. It has been concluded that the instabilities are primarily caused by the migration of halogen ions at high pressures, as previously confirmed (Supplementary Fig. 26).
Therefore, (MDABCOBr)–NH4(BF4)3 single crystal detectors are expected for high-performance X-ray imaging owing to their remarkable sensitivity, stability, and low detection limit. The X-ray imaging process involves the movement of the image object using an X–Y shift table positioned between the X-ray source and the single-pixel detector. The acquisition of X-ray images is achieved by capturing the transmitted X-ray photons versus location (x, y)40. Finally, as shown in Fig. 3j, the imaging of the metal radiation logo is clearly displayed.
Biotoxicity and flexibility
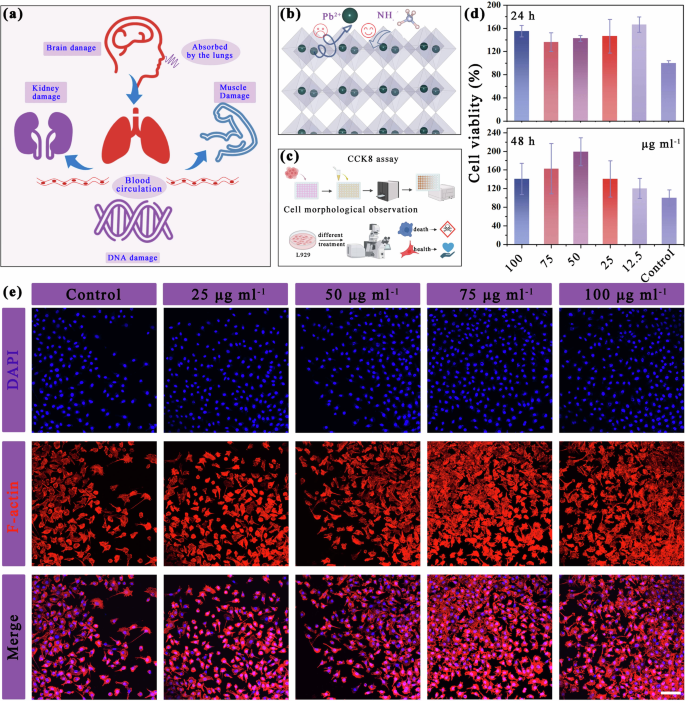
Metal halide perovskites are popular in the field of photoelectronics; however, ensuring their biosafety remains challenging due to the presence of heavy metals. For example, Pb2+ can enter the bloodstream through ingestion, breathing, and skin contact, distribute to soft tissues such as lungs, heart, liver, kidneys, and brain, and accumulate in bones. The accumulation of Pb2+ can regulate fundamental physiological functions in the human body, as well as interfere with heme activity, which is essential for oxygen binding in the blood. Moreover, these Pb2+ have the potential to disrupt enzyme and receptor function within soft tissues (Fig. 4a)41,42. Unfortunately, there is currently insufficient evidence to support the biocompatibility of pseudohalide–MFPs. Therefore, it is imperative to conduct a systematic investigation into the biocompatibility of pseudohalide–MFPs. This study investigates the cytocompatibility of (MDABCOBr)–NH4(BF4)3 crystals. The extracts of varying concentrations of this material were placed in 96-well plates (Supplementary Fig. 27). The L929 fibroblasts were selected for the cell viability test43. Fig. 4b, c presents the cells viability data and fluorescence images at different incubation times. It can be seen that the growth ability of L929 cells can be enhanced in the content range of 12.5–100 µg ml−1, which is consistent with the results reported previously (Fig. 4d, e)44,45. The cells viability also remained better with the extension of the incubation time. In contrast, the study conducted by Benmessaoud et al. revealed that even at concentrations as low as 50 µg ml−1, MAPbI3 still triggers a significant level of cell death (from 10% to 30%). Furthermore, it was observed that concentrations up to 200 µg ml−1 could result in the death of over half of the cell population46.
a Illustration of the lead toxification in the human body. b Schematic of replacing Pb2+ with NH4+ as the B-site cation. c Flow chart of CCK8 experiment. d Cell viability of (MDABCOBr)-NH4(BF4)3 over 24 and 48 h calculated as the fraction of total living cells. e (MDABCOBr)-NH4(BF4)3 images of the live/dead staining of planktonic S. aureus with different treatments.
The utilization of heavy and bulky equipment can lead to significant discomfort, while prolonged usage exacerbates the psychological and physical burden on the user. Hence, employing lightweight and flexible imaging devices proves to be an effective means of alleviating this load. Furthermore, flexible devices have a greater propensity for bending and conforming to biological surfaces than conventional solid devices. Leveraging the exceptional X-ray detection performance and biocompatibility of (MDABCOBr)–NH4(BF4)3 single crystals, we endeavored to fabricate a wearable X-ray detector (Fig. 5a). Poly(vinylidene fluoride) (PVDF) was selected as a flexible substrate because of its suitable physicochemical properties and mechanical properties. As shown in the Supplementary Fig. 28, we measured the stability of PVDF at high dose rates and high humidity conditions, confirming its excellent resistance to radiation and the environment, which ensures that our equipment will not suffer from matrix failure.
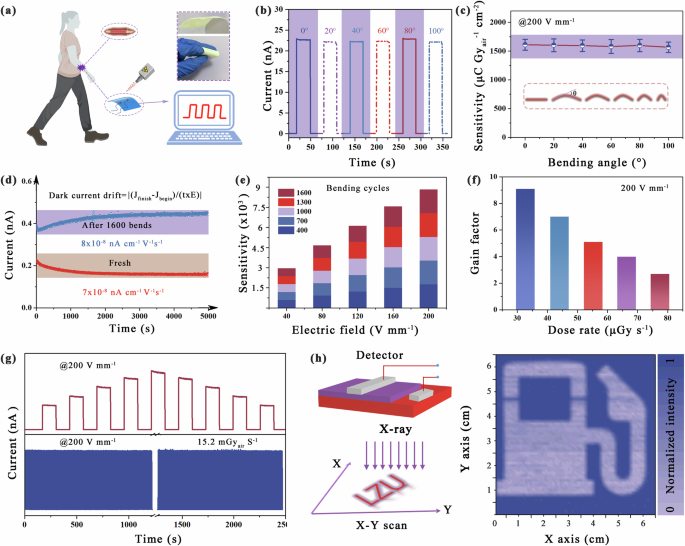
a Schematic diagram of the flexible X-ray device laminated to the human body (Inset: the flexibility schematic). b Current response and c sensitivity of the device at different bending angles. d Dark current drift of the device before and after 1600 bends. e Sensitivity of the device under different electric fields and bending times. f Gain factor of the device at different dose rate under an electric field of 200 V mm−1. g The operating stability of flexible devices under different doses and long-term switching. h Imaging of flexible devices.
The fabricated device has good homogeneity, and the X-ray excitation current is highly consistent in different regions (Supplementary Figs. 29 and 30). As shown in Fig. 5b, c, the corresponding response current, as well as sensitivity, remains almost constant for different radii of curvature, showing excellent device robustness. In addition, we test the flexible device in continuous bending cycles, where the bending radius of each time is defined as 10 mm. Fig. 5d shows that there is only a negligible change (from 7.0 × 10−8 nA cm−1 s−1 V−1 to 8.0 × 10−8 nA cm−1 s−1 V−1) in the dark current drift of the (MDABCOBr)–NH4(BF4)3 device after 1600 bends. As shown in Fig. 5e, the sensitivity remained essentially constant even after 1600 bends at different electric fields.
In addition, the gain factor of the flexible (MDABCOBr)–NH4(BF4)3 device reaches 9.1 at 32.67 uGyair s−1 under an electric field of 200 V mm−1, which indicates that the flexible pseudohalide–MFPs has great potential as a sensitive X-ray detection device. Interestingly, the gain factor gradually decreases to 2.7 as the dose rate increases to 77.44 uGyair s−1, which can be attributed to the progressive filling of charge carriers into shallower traps, leading to smaller gains under higher irradiation intensities (Fig. 5f)2. In particular, the continuous and fast switching current under the electric field of 200 V mm−1 is not attenuated, indicating the device has extraordinary operation stability (Fig. 5g). Simultaneously, we analyzed the storage stability of (MDABCOBr)–NH4(BF4)3 materials and devices in air. Supplementary Figure 31a shows that the material maintained its structure after 10 months in air. The corresponding device is also retested unaffected by the attack of oxygen and water molecules in the air, and the change from the initial performance is almost negligible (Supplementary Fig. 31b, c). The ultra-stability against humidity and oxygen is due to the protection of molecular perovskite octahedra by polar enhanced DABCOBr2+ ions and the enhanced interaction between the various components of the optimized structure. To demonstrate the imaging capability of the flexible pseudohalide–MFPs X-ray detector at a low dose rate, we successfully captured an image of a “gasolene station” logo constructed with a metal base using an X-ray dose rate of 1.2 uGyair s−1 (this process is consistent with that described in Fig. 3f). It is evident that the clarity of the logo and the high signal-to-noise ratio are readily apparent (Fig. 5h). These achievements indicate that flexible pseudohalide–MFPs with high stability, outstanding bending performance, and imaging capability, which are promising in wearable devices for mobile health.